Ukrainian version of FRAX in fracture risk assessment for women with rheumatoid arthritis
Authors:
Povoroznyuk Vladyslav 1; Grygorieva Nataliia 1; Ivanik Oksana 2; Bystrytska Maryna 1; Dzerovych Nataliia 1; Povorozniuk Vasyl 1; Musiienko Anna 1; Zaverukha Nataliia 1
Authors place of work:
D. F. Chebotarev Institute of Gerontology of the National Academy of Medical Sciences of Ukraine, Kyiv, Ukraine
1; РІ of the Lviv Regional Council “Lviv Regional Clinical Hospital”, Lviv, Ukraine
2
Published in the journal:
Clinical Osteology 2020; 25(3): 120-127
Category:
Přehledové články
Summary
Nowadays, the numerous studies confirm an increased fractures risk in the rheumatoid arthritis (RA) patients. FRAX is informative tool for fracture risk assessment; however, data about its use in patients with RA are limited. The aim of this study was to determine the 10-year probability of major osteoporotic fractures and hip fractures of the RA female patients by means of the Ukrainian FRAX version. We have performed a cross-sectional case-control study, examining 794 women aged 40–89 years that were divided in two groups: І (n = 397) – healthy subjects without RA and ІІ (n = 397) – patients with a verified RA diagnosis. The appraisal of major osteoporotic fracture risk and hip fracture one was made according to the Ukrainian model of FRAX without and with bone mineral density (BMD) measurement. This study demonstrated that the RA patients have significantly higher FRAX values, compared to healthy subjects both in the total group and in different age subgroups. There was a strong correlation between the FRAX values with/out the BMD in the RA patients (r = 0.75). About 40 % of the RA patients require antiosteoporotic treatment even without DXA results, based exclusively on the FRAX values, and about 47 % more require a revision of fracture risk after the BMD measurement. Additionally, only 7 % of patients require osteoporosis treatment revision after BMD measurement. All the above-mentioned facts should be considered while managing this category of patients in order to reduce the risk of osteoporotic fractures.
Keywords:
rheumatoid arthritis – FRAX – fracture risk – 10-year probability of osteoporotic fractures
Introduction
The rheumatoid arthritis (RA) is one of the most common rheumatic conditions [1,2] leading to a consistent disability and increased mortality rate among women as well as among men. The systemic osteoporosis is a frequently-occurring comorbid pathology in the RA patients, associated with the bone loss due to a chronic inflammation with an autoimmune component [3,4] and use of some anti-rheumatic medications (i.e. glucocorticoids) [5].
The numerous studies are confirming an increased fracture risk in the RA patients [6–8]. The recently published metaanalysis which includes 13 studies [6] demonstrated a significantly higher fracture risk in the RA patients compared to the healthy subjects: relative risk (RR) = 2.25; 95 % СІ = 1.76–2.87, both for women: RR = 1.99; 95 % CI = 1.58–2.50 and for men: RR = 1.87; 95 % CI = 1.48–2.37). The authors indicate an increased risk of major osteoporotic fractures – of vertebral: RR = 2.93; 95 % СІ = 2.25–3.83 and hip ones: RR = 2.41; 95 % CI = 1.83–3.17 – in the RA patients compared to the healthy subjects.
At present, the various studies confirm that increased fracture risk in the RA patients is attributed to the decreased bone mineral density (BMD) and other factors affecting the bone micro - and macro-architecture and bone remodeling rate. The timely assessment of fracture risk is extremely topical, in terms of screening and determination of chronic autoimmune management tactics, namely the RA [3,4].
For the RA patients, the determination of fracture risk is based on the complex evaluation of BMD values and the Fracture Risk Assessment Tool (FRAX®) questionnaire, calculating a 10-year probability (risk) of major osteoporotic fractures (hip, shoulder, and forearm fractures and clinically relevant vertebral fractures) and, separately, of hip fracture for the subjects of 40 years and over [9,10]. In Ukraine, there is a national FRAX version [11], developed especially for the Ukrainian population, and the recently obtained threshold values for women, required for the timing of antiosteoporotic treatment start and the arrangement of additional examination [12]. Despite these new developments, there is a lack of data on the informative value of the Ukrainian FRAX® model in the RA patients. This underlying idea prompted the present research.
The aim of this study is to determine the 10-year probability of major osteoporotic fractures and hip fractures of the RA female patients by means of the Ukrainian FRAX version.
Materials and methods
We have performed a cross-sectional case-control study, examining 794 women aged 40–89 years at the SI “D.F. Chebotarev Institute of Gerontology by the NAMS of Ukraine” (Kyiv) and the “Lviv regional clinical hospital”, municipal establishment of Lviv regional council (Lviv).
The study was conducted according to a unified protocol at both centers, approved by the Committee of Ethics at the SI “D. F. Chebotarev Institute of Gerontology by the NAMS of Ukraine” (27. 5. 2016, protocol № 5). All of the women signed a voluntary informed consent to the participation and the specific study procedure use.
For the sake of analysis, the examined subjects were divided in two groups: І (control group, n = 397) – practically healthy subjects without RA and ІІ (main group, n = 397) – patients with a verified RA diagnosis. In terms of age, the patients did not differ significantly (in the control and main group, respectively: 60.6 ± 12.3 and 59.6 ± 9.9 years; t = 1.22; р = 0.24).
Using the routine methods, we have calculated the main anthropometric parameters (height and weight), body mass index (BMI) by the conventional formula: (weight (kg) / height2 (m2). The women of control group had significantly higher height parameters compared to the RA patients (respectively: 1.62 ± 0.07 and 1.61 ± 0.07 m; t = 3.34; р = 0.001), though their weight parameters did not differ (71.3 ± 15.0 and 70.8 ± 14.2 kg; t = 0.51; р = 0.61) and their BMI (23.2 ± 5.7 and 23.2 ± 4.6 kg/m2; t = 0.09; р = 0.97) did not differ either.
Among the subjects, 84 % women were in the postmenopausal age (respectively: 81.4 % in the І group and 86.9 % in the ІІ group). In the I and II groups, the mean age of menopause was respectively: 49.4 ± 3.8 and 47.8 ± 3.8 years (t = 5.5; р = 0.0001); the postmenopausal duration was 14.0 [5.0–23.0] and 12.0 [6.0–19.0] years (Z = 0.87; р = 0.38).
We have performed analysis of subgroups in terms of age (40–44, 45–49, 50–54, 55–59, 60–64 and 65–69 years) and menopausal status (premenopause, 1–4, 5–9, 10–14, 15–19, 20 and more years of postmenopausal period).
The appraisal of major osteoporotic fracture risk in general (FRAX-MOF) and hip fractures in particular (FRAX-HF) was made according to the Ukrainian model of FRAX, present at the official FRAX webpage (https:// www.sheffield.ac.uk/FRAX), either without (FRAX-MOF-BMI and FRAX-HF-BMI) or with (FRAX-MOF-BMD and FRAX-HF-BMD) consideration to the dual-energy X-ray absorptiometry (DXA) findings. Furthermore, we have calculated the ratio of women requiring an additional determination of the BMD parameters or osteoporosis treatment (with/out the DXA evaluation) according to the nationally-developed recommendations.
The necessity of antiosteoporotic treatment in terms of the DXA parameters was evaluated according to the WHO recommendations (1994) [13], whose suggested osteoporosis criterion is the T-score ≤ -2.5 (reduced BMD > 2.5 SD of the reference values). In order to detect the need for the additional examination and treatment, we have selected 3 subgroups according to the recommended intervention thresholds by the Ukrainian model of FRAX [12]:
- 10-year FRAX-MOF probability below which one should not consider either treatment or the additional DXA examination (lower evaluation threshold)
- 10-year FRAX-MOF probability over which one should consider treatment, irrespective of the BMD values (upper evaluation threshold)
- 10-year FRAX-MOF probability at which one should consider additional BMD measurement (values in between the lower and upper evaluation thresholds)
The determination of hip BMD values, used in order to calculate the FRAX values, as well as of lumbar spine BMD, along with the T-score detection at the above mentioned spinal regions, was made by means of the Prodigy, Discovery (2010) and Lunar General Electric (2005) densitometers.
The analysis of findings was performed by means of «Statistica 10.0» software. The sample’s relevance in terms of normal distribution principle was checked by Shapiro-Wilk’s test. As there is a varied character of data distribution, the findings were presented as mean values (M) and standard deviation (SD) (in terms of normal distribution principle), as well as median (Ме) and the lower and upper quartiles (25Q–75Q) in case of a non-convergence. The findings of two independent samples were compared with a two-sample Student criterion (t) for the independent samples or Mann-Whitney test (in case of convergence/non-convergence to the normal distribution principle). The correlation analysis was performed by means of Pearson correlation (r). In order to evaluate the distinctions of frequencies in both independent samples, we have used χ2-criterion. The findings were considered significant with р < 0.05.
Results
According to the Ukrainian FRAX version, without BMD values (FRAX-MOF-BMI), the comparison of FRAX-MOF parameters detected significantly higher values among the RA female contrasteding with the control group > 8.4 (5.9–13.0) and 6.2 (3.3–9.1) %; Z = 9.2; р = 0.0001. Similar distinctions were revealed while analyzing the FRAX-HF-BMI values, amounting to 1.3 (0.4–2.9) % in the I group, 2.1 (1.0–4.4) % in the II group, resp. (Z = 7.3; р = 0.0001).
The analysis of FRAX-MOF values, with an account of BMD values, has also corroborated the above-mentioned findings. In the RA patients and control subjects, respectively, the FRAX-MOF-BMD parameter amounted to 9.1 (6.2–16.0) and 5.8 (3.4–9.2) %; Z = 10.2; р = 0.0001, while the FRAX-HF-BMD parameter amounted to 2.0 (0.7–5.8) and 1.1 (0.4–2.6) %; Z = 6.5; р = 0.0001.
The exploration of the FRAX parameter with/out BMD consideration showed a strong significant association both in the control group subjects (figure 1А) and the RA patients (figure 1B).
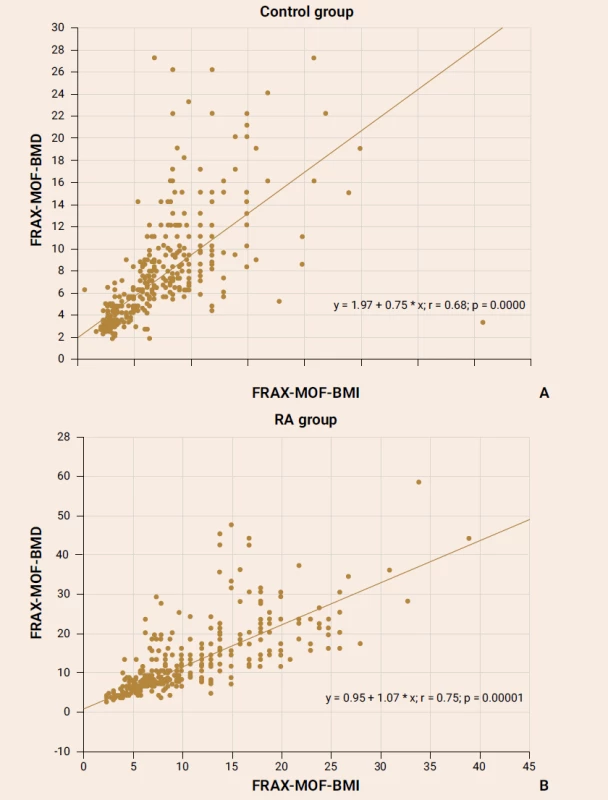
The significant positive correlation between the FRAX-HF-BMI and FRAX-HF-BMD parameters was reported in the RA female patients (r = 0.59; p = 0.0001) as well as in healthy subjects (r = 0.58; p = 0.0001).
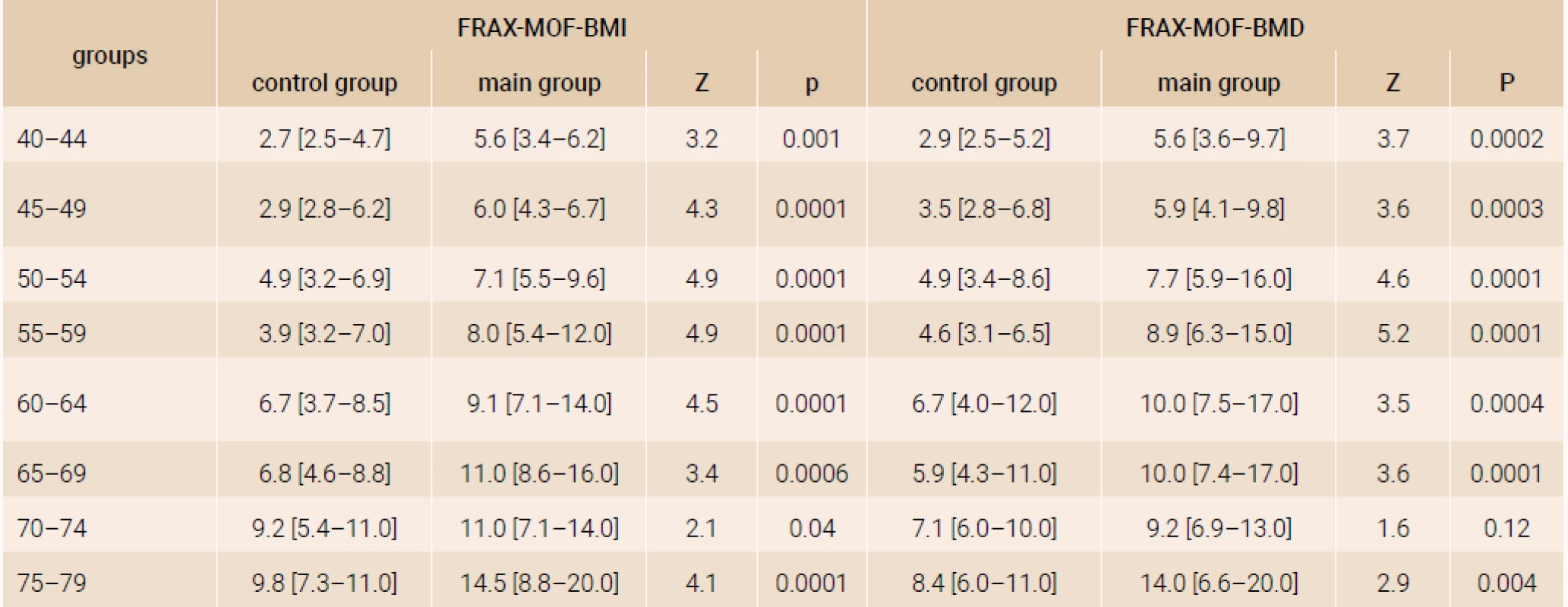
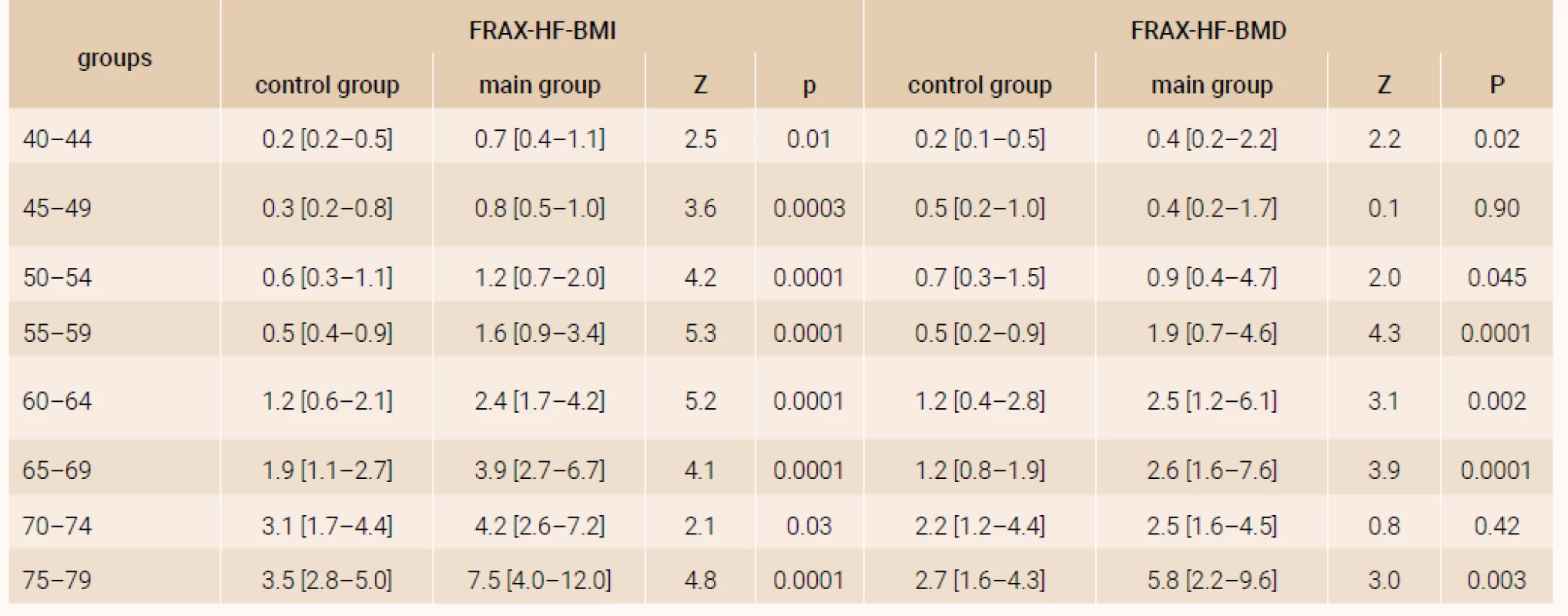
The analysis of a 10-year probability of major osteoporotic fracture and hip fracture risk was performed in the age subgroups distributed by 5-year intervals and confirmed significant distinctions of FRAX parameters both with and without DXA (table 1 and table 2), except for the parameters of women of 70–74 years (FRAX-HF - BMD).
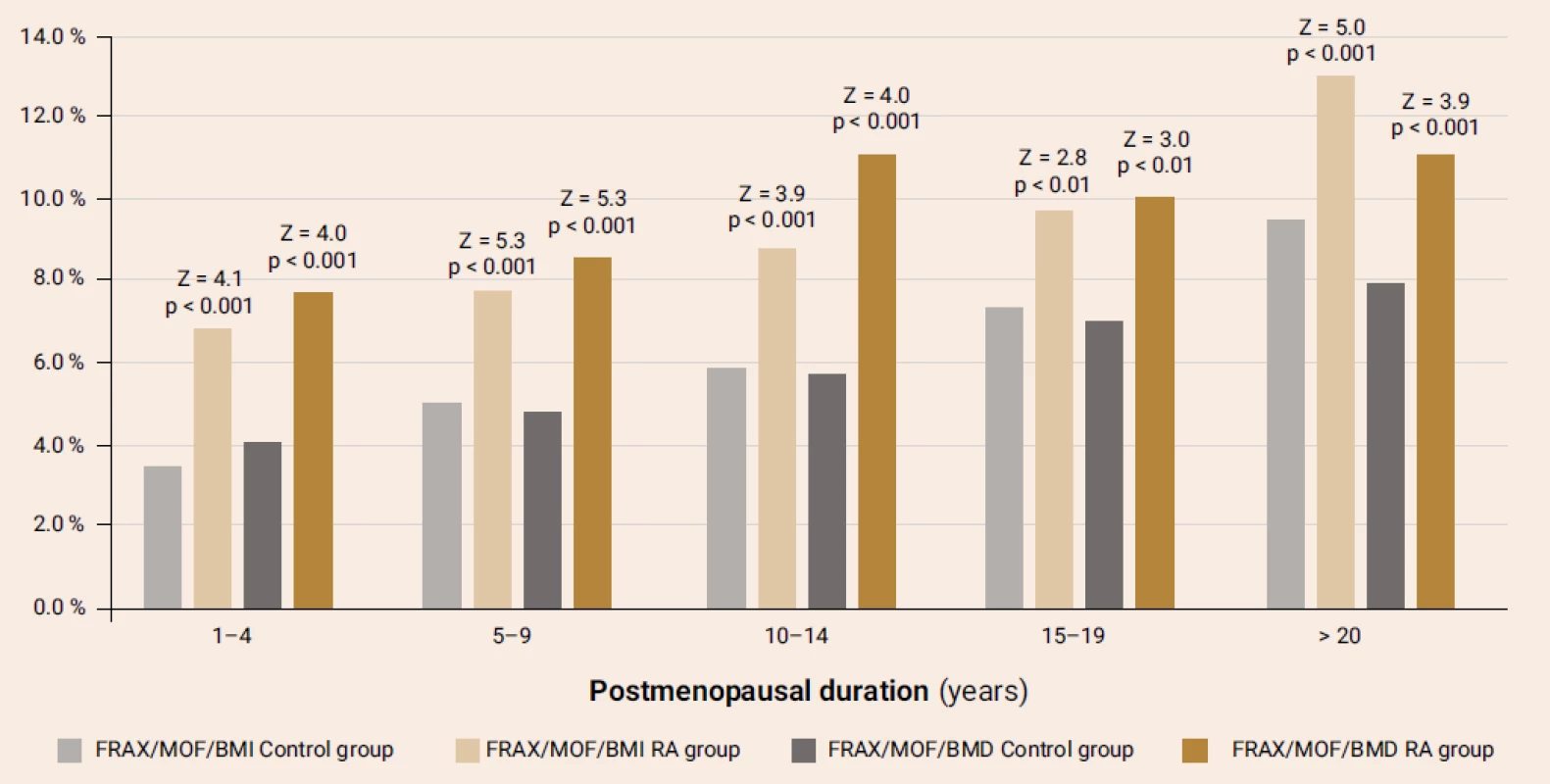
The comparison of FRAX-MOF parameters in terms of the RA presence and postmenopausal duration (figure 2) revealed significantly higher parameters in the RA patients compared with the respective parameters of control group, irrespective of the BMD use while calculating FRAX. Similar distinctions were found for the hip fracture probability.
By comparing the frequency of low-energy fractures in the groups, we have managed to revealed their greater prevalence (34.0 %) in the RA women rather than in the control group (17.6 %; χ2 = 27.8; р < 0.001).
The using of glucocorticoid was significantly higher among the RA patients (63.5 %) compared to the control group parameters (5.1 %; р < 0.0001). The hip fractures in subject parents were reported by 6.1 % of the RA patients and 8.1 % of the control group subjects (р > 0.05). The smoking habit was typical of 1.51 % of the RA patients and 1.76 % of healthy subjects (р > 0.05), while 3.5 and 3.3 % (р > 0.05) of subjects were consuming alcohol in the doses higher than those considered safe, respectively.
We have performed our own analysis of the need for an additional examination and antiosteoporotic treatment using the index of 10-year probability of major osteoporotic fractures without the BMD consideration and found that among the women of control group only 14.1 % had higher values than those imposed by the upper intervention threshold, according to the Ukrainian FRAX version, and required treatment even without DXA results, while among the RA patients this parameter amounted to 40.3 % (χ2 = 31.0; р < 0.001). The women of control group who did not require additional examination or treatment, according to the FRAX parameters, made up 21.2 % of the subjects from this group, while among patients with RA this parameter was 1.8 %.
Our analysis of the osteoporosis frequency by DXA according to T-score (evaluated at the lumbar spine and/or hip level) among the postmenopausal women showed that the prevalence of osteoporosis among the RA patients was significantly higher (67 %), compared to the control group values (30 %; χ2 = 47.7; р < 0.001).
The factoring of BMD parameter into the calculation of a 10-year probability of major osteoporotic fractures revealed that 22 % of control group subjects and but 4.4 % of the RA patients do not require any treatment of osteoporosis, while half the examined subjects (57.1 % in the І group and 48.1 % in the ІІ group) remain in the “moderate risk” zone, according to the FRAX index. The treatment is required by twice as many RA patients (47.5 %) compared to the control subjects (20.6 %).
Discussion
The rheumatoid arthritis (RA) is still one of the most common chronic inflammatory articular conditions [1,2], characterized by a progressing symmetrical inflammation in the afflicted joints. Its prevalence varies within the range of 0.4–1.3 %, the condition predominating in women, and under certain geographical, social and other factors.
The systemic osteoporosis and its complications – fragility fractures – are among the most frequent comorbidities in the RA patients, this fact being attributed to the negative influence of inflammation on the bone tissue, increased rate of inflammatory cytokines and circulating auto-antibodies [3,4]. The negative influence is reinforced by the glucocorticoid use, which are rather often prescribed, especially with the high RA activity [3,5]. The increased risk of low-energy (fragility) fractures complicates the course of RA, promotes disability and increased mortality.
In the systematic review and metaanalysis, published in 2018 and composed of 25 studies [8], the combined incidence index of overall and fragility fractures came up to 33.00 (95 % CI: 18.39–59.21) and 15.31 (95 % CI: 10.43–22.47) per 1 000 person-years, respectively. The fracture risk was higher for women rather than men (31.03 vs. 23.75 per 1000 person -years). The RA patients had a higher risk of all fractures (RR = 1.52; 95 % CI: 1.07–2.14) and low-energy fractures (RR = 1.61; 95 % CI: 1.44–1.79); the incidence indices of vertebral, hip, shoulder and forearm fractures being, respectively: 7.51 (95 % CI: 3.27–17.23), 4.33 (95 % CI: 2.26–8.27), 1.86 (95 % CI: 1.36–2.53) and 3.40 (95 % CI: 2.27–5.10) per 1 000 person -years. One should emphasize that the fracture predictors might have been either the well-known risk factors of osteoporosis or the RA-associated factors.
At present, in order to assess the fracture risk factors and to prevent the fractures in a timely manner, they are effectively using DXA and the FRAX questionnaire across the world. Both methods have their own informative value, required for the decision-making on the initiation of antiosteoporotic treatment. The FRAX questionnaire, in particular, includes such important items as the RA (presence of the condition (item 9) and glucocorticoid treatment (item 8).
In Ukraine, the FRAX algorithm is being actively used in order to evaluate the osteoporotic fracture risk since 2010; the Ukrainian FRAX model was posted on the official FRAX webpage in 2016 [10], with intervention and additional examination thresholds being added to this model in 2019 [12]. At the moment, the Ukrainian FRAX model is routinely used in the clinical practice; however, the experience of FRAX being used by the RA patients starts being accumulated in Ukraine and other countries [6,14–19].
In 2011, the International Society for Clinical Densitometry (ISCD) and the International Osteoporosis Foundation (IOF) published their concerted position as to the FRAX being used by the RA patients [20], concluding that the FRAX may underestimate the risk of fracture with the RA and functional status disorders. However, there are studies corroborating a strong correlation of the FRAX and disease duration and fracture risk in the RA patients, as well as the patients’ health, functional RA class and risk of clinically, though not morphometrically, confirmed vertebral fractures.
The other recommendations of the above-mentioned societies [21] stress the urgency of considering a dose-dependent glucocorticoid influence while assessing the osteoporotic fracture risk. The use of prednisone (or its equivalent) at a daily dose of 2.5–7.5 mg does not require the FRAX value correction; however, when the glucocorticoid dose rises or drops, one should revise it. In the future, the study findings enable the elaboration of the FRAX correction coefficients depending on the glucocorticoid dose [22], which gained a high degree of acceptance by the clinical practice.
This cross-sectional case-control study was aimed at the analysis of a 10-year probability of major osteoporotic and hip fractures for the RA patients by means of the Ukrainian version of the FRAX algorithm. The study was performed according to the unified protocol at 2 centers.
We have reported significantly higher FRAX values for the major osteoporotic and hip fractures of the RA patients compared to the control subjects in the total group and separate age subgroups, both with the BMD parameters factored in/out of the FRAX calculations. Furthermore, among the RA patients there is a significantly higher prevalence of subjects requiring the additional DXA examinations and antiosteoporotic treatment, even with no account of DXA.
The informative value of FRAX and DXA (osteoporosis being diagnosed by the T-score of ≤ -2.5 SD or the Z-score of ≤ -2.0 SD, according to the WHO recommendations) were compared in the retrospective multicenter study involving 479 RA patients [16]. The FRAX calculations were made with and without the BMD measurement involved. According to the NOF criteria [23], the fracture risk was considered high whenever the FRAX-MOF was ≥ 20 % for major osteoporotic fractures or the FRAX-HF was ≥ 3 % for hip fractures, respectively. The prevalence of subjects requiring the antiosteoporotic treatment, according to the FRAX criteria (with/out BMD measurements) and DXA findings, amounted to 47.2 %, 61 % and 33.4 %, respectively. In a study providing the findings similar to ours, the prevalence of RA patients requiring treatment, according to the above-mentioned criteria, accounted for 40.3 %, 67 % and 47.5 %.
Another study [6] delves into the FRAX’s informative value in 200 RA patients aged over 40 years, women’s prevalence being 77.5 % of patients. The authors confirm a lack of confirmed distinctions of the FRAX values with/out the BMD measurements involved. We have received no significant distinctions of the FRAX values with/out the additional DXA results either. Among the control group subjects, the prevalence of women requiring treatment with/out the BMD measurements made 14.1 and 20.6 %, respectively; while among the RA patient group, the prevalence of women was 40.3 and 47.5 %, respectively.
Another recently published study [15] recruiting 232 RA patients aged 40–90 years (most of them being women) analyzed the impact of the disease-associated factors on the FRAX values. 46 % of patients reported present osteoporotic fractures, most of them being vertebral ones (87 %). 57 %, 25 % and 18 % had a high, moderate and low 10-year probability of major osteoporotic fractures. The respective prevalence for the hip fracture risk was 51 %, 34 % and 15 %, respectively. The key factors associated with a 10-year risk of major osteoporotic and hip fractures were the disease duration, postmenopausal duration, disease activity and deteriorated health. Among the RA patients of this study, the prevalence of fractures was somewhat lower (34 %) though the major osteoporotic fracture risk rate for the RA patients demonstrates a higher (compared to the above-mentioned study [15]) frequency of medium-to-high risk patients (but for 1.8 % of patients not exposed to DXA and 4.4 % of subjects without the BMD measured require additional examination or treatment, and belong to the “low risk” group, according to the FRAX).
The study involving 50 RA patients from the Danish rheumatology register (DANBIO) [19] demonstrated that the FRAX-MOF parameter calculated with/out BMD amounted to 25.8 ± 18.6 and 22.9 ± 15.8 %; however, as the authors note, the urgency of antiosteoporotic treatment, according to the NOF recommendations [23], depended on the BMD only in 4 % of patients. Our study has also corroborated a high correlation of two FRAX models (with/out the BMD measured) in the RA patients (r = 0.75), and the urgency of osteoporosis treatment revision according to DXA only in 7.2 % of patients.
Conclusions
The study we’ve performed proves that the RA female patients have significantly higher FRAX values, compared to healthy subjects both in the total group and in various age subgroups. There is a strong correlation between the FRAX values with/out the BMD measurements in the RA patients (r = 0.75). About 40 % of the RA patients require antiosteoporotic treatment even without DXA results, based exclusively on the FRAX values, and about 47 % more require a revision of fracture risk after the BMD measurement. Only 7.2 % of patients requiring osteoporosis treatment revision after DXA. All the above-mentioned facts should be considered while managing this category of patients in order to reduce the risk of osteoporotic fractures.
Disclaimer. The authors report no conflict of interests; they did not receive any financial support, researcher fees or other forms of remuneration from the individuals or institutions.
Nataliia Grygorieva, MD, PhD | crystal_ng@ukr.net | www.geront.kiev.ua
Received | Doručené do redakcie | Doručeno do redakce 10. 8. 2020
Accepted | Prijaté po recenzii | Přijato po recenzi 31. 8. 2020
Zdroje
-
Sparks JA. Rheumatoid Arthritis. Ann Intern Med 2019; 170(1): ITC. 1–16. Dostupné z DOI: <http://doi/10.7326/AITC201901010>.
-
Lin YJ, Anzaghe M, Schülke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020; 9(4): pii. E880. Dostupné z DOI: <http://dx.doi.org/10.3390/cells9040880>.
-
Adami G, Saag KG. Osteoporosis Pathophysiology, Epidemiology, and Screening in Rheumatoid Arthritis. Curr Rheumatol Rep 2019; 21(7): 34. Dostupné z DOI: <http://dx.doi.org/10.1007/s11926–019–0836–7>.
-
Adami G, Fassio A, Rossini M et al. Osteoporosis in Rheumatic Diseases. Int J Mol Sci 2019; 20(23): 5867. Dostupné z DOI: <http://dx.doi.org/10.3390/ijms20235867>.
-
Chotiyarnwong P, McCloskey E. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol 2020; 16 : 437–447. Dostupné z DOI: <http://dx.doi.org/10.1038/s41574–020–0341–0>.
-
Wang Y, Hao YJ, Deng XR et al. Risk factors for bone mineral density changes in patients with rheumatoid arthritis and fracture risk assessment. Beijing Da Xue Xue Bao Yi Xue Ban 2015; 47(5): 781–786>.
-
Xue AL, Wu SY, Jiang L et al. Bone fracture risk in patients with rheumatoid arthritis. A meta-analysis. Medicine 2017; 96(36): e6983. Dostupné z DOI: <http://dx.doi.org/10.1097/md.0000000000006983>.
-
Jin S, Hsieh E, Peng L et al. Incidence of fractures among patients with rheumatoid arthritis: a systematic review and meta-analysis. Osteoporos Int 2018; 29(6): 1263–1275. Dostupné z DOI: <http://dx.doi.org/10.1007/s00198–018–4473–1>.
-
Kanis JA, Harvey NC, Johansson H et al. A decade of FRAX: how has it changed the management of osteoporosis? Aging Clin Exp Res 2020; 32 : 187–196. Dostupné z DOI: <http://dx.doi.org/10.1007/s40520–019–01432-y>.
-
Kanis JA, Harvey NC, Cyrus Cooper C et al. A systematic review of intervention thresholds based on FRAX. A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos 2016; 11 : 25. Dostupné z DOI: <http://dx.doi.org/10.1007/s11657–016–0278-z>.
-
Povoroznyuk VV, Grygorieva NV, McCloskey et al. Application of FRAX to determine the risk of osteoporotic fractures in the Ukrainian population. International Journal of Osteoporosis and Metabolic Disorders 2018; 11(1): 7–13. Dostupné z DOI: <http://dx.doi.org/10.3923/ijom.2018.7.13>.
-
Povoroznyuk VV, Grygorieva NV, Kanis JA et al. Ukrainian FRAX: criteria for diagnostics and treatment of osteoporosis. Pain Joints Spine 2019; 9(4): 9–16. Dostupné z DOI: <http://dx.doi.org/10.22141/2224–1507.9.4.2019.191921>.
-
WHO scientific group on the assessment of osteoporosis at primary health care level. Summary Meeting Report Brussels, Belgium, 5–7 May 2004. Informace dostupné z WWW: <https://www.who.int/chp/topics/Osteoporosis.pdf>.
-
Lai EL, Huang WN, Chen HH et al. Ten-year fracture risk by FRAX and osteoporotic fractures in patients with systemic autoimmune diseases. Lupus 2019; 28(8): 945–953. Dostupné z DOI: <http://dx.doi.org/10.1177/0961203319855122>.
-
Phuanudom R, Lektrakul N, Katchamart W. The association between 10-year fracture risk by FRAX and osteoporotic fractures with disease activity in patients with rheumatoid arthritis. Clinical Rheumatology 2018; 37 : 2603–2610. Dostupné z DOI: <http://dx.doi.org/10.1007/s10067–018–4218–8.
-
Choi ST, Kwon SR, Jung JY et al. Prevalence and Fracture Risk of Osteoporosis in Patients with Rheumatoid Arthritis: A Multicenter Comparative Study of the FRAX and WHO Criteria. J Clin Med 2018; 7(12): pii. E507. Dostupné z DOI: <http://dx.doi.org/10.3390/jcm7120507>.
-
Meng J, Li Y, Yuan X, Lu Y. Evaluating osteoporotic fracture risk with the Fracture Risk Assessment Tool in Chinese patients with rheumatoid arthritis. Medicine (Baltimore) 2017; 96(18): e6677. Dostupné z DOI: <http://dx.doi.org/10.1097/MD.0000000000006677>.
-
Povoroznyuk VV, Grygorieva NV, Ivanik OC. 10-year probability of major osteoporotic fractures in women with rheumatoid arthritis by the Ukrainian FRAX model. Trauma 2020; 21(2): 1–7. Dostupné z DOI: <http://dx.doi.org/10.22141/1608–1706.2.21.2020.202227>.
-
Elde KD, Madsen OR. FRAX 10-yr fracture risk in rheumatoid arthritis—assessments with and without bone mineral density may lead to very different results in the individual patient. Journal of Clinical Densitometry 2019; 22(1): 31–38. Dostupné z DOI: <http://dx.doi.org/10.1016/j.jocd.2018.10.007>.
-
Broy SB, Tanner SB. FRAX(®) Position Development Conference Members. Official Positions for FRAX® clinical regarding rheumatoid arthritis from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX®. J Clin Densitom 2011; 14(3): 184–189. Dostupné z DOI: <http://dx.doi.org/10.1016/j.jocd.2011.05.012>.
-
Leib ES, Saag KG, Adachi JD et al; FRAX(®) Position Development Conference Members. Official Positions for FRAX(®) clinical regarding glucocorticoids: the impact of the use of glucocorticoids on the estimate by FRAX(®) of the 10 year risk of fracture from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX(®). J Clin Densitom 2011; 14(3): 212–219. Dostupné z DOI: <http://dx.doi.org/10.1016/j.jocd.2011.05.014>.
-
Kanis JA, Johansson H, Oden A et al. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int 2011; 22(3): 809–816. Dostupné z DOI: <http://dx.doi.org/10.1007/s00198–010–1524–7>.
-
Cosman F, de Beur S J, LeBoff MS et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International 2014; 25(10): 2359–2381. Dostupné z DOI: <http://doi: 10.1007/s00198–014–2794–2>.
Štítky
Biochemie Dětská gynekologie Dětská radiologie Dětská revmatologie Endokrinologie Gynekologie a porodnictví Interní lékařství Ortopedie Praktické lékařství pro dospělé Radiodiagnostika Rehabilitační a fyzikální medicína Revmatologie Traumatologie OsteologieČlánek vyšel v časopise
Clinical Osteology

2020 Číslo 3
Nejčtenější v tomto čísle
- Neurogenic heterotopic ossification
-
23rd Congress of Slovak and Czech Osteologists
September the 17th–19th 2020 | Double Tree by Hilton | Bratislava | Slovakia - Clinical osteology – certificated work activity
- Selected biomarkers associated with atherosclerosis and bone metabolism
